Audio-Visual Stimulation and the hypothalamic-pituitary-adrenal axis
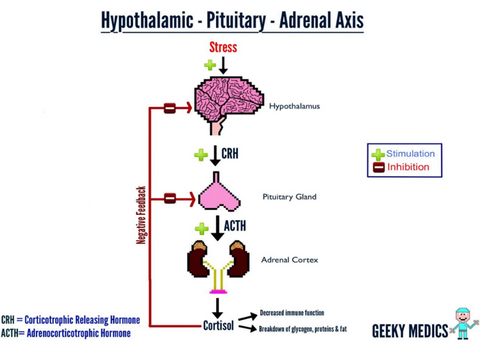
The adrenal axis (hypothalamic-pituitary-adrenal axis) is a crucial neuroendocrine system referring to a complex set of interactions and feedback loops between the hypothalamus, pituitary, and adrenal glands. This system regulates the body’s response to stress, immune function, energy expenditure, mood, emotions, and libido.
Components of the HPA Axis
Hypothalamus: This small region at the base of the brain detects stress and releases corticotropin-releasing hormone (CRH). It plays a key role in maintaining homeostasis and regulating various bodily functions, including temperature and hunger.
Pituitary Gland: Triggered by CRH, the pituitary gland releases adrenocorticotropic hormone (ACTH) into the bloodstream. This hormone stimulates the adrenal glands to produce cortisol.
Adrenal Glands: Located atop the kidneys, these glands respond to ACTH by releasing cortisol, the primary stress hormone. Cortisol helps the body manage stress by increasing blood sugar levels, suppressing the immune system, and influencing metabolism.

Function of the HPA Axis
The HPA axis is activated in response to physical or psychological stressors. When a stressor is perceived, the hypothalamus releases CRH, leading to a cascade of hormonal signals that ultimately result in increased cortisol production. This process prepares the body for a fight-or-flight response, providing the necessary energy and resources to cope with the stressor. Stressors can both be from disease, body stress from running a marathon, and psychological stress from everything imaginable. When stress, whether physical, cognitive, or emotional becomes more than the pituitary gland can handle, it shuts down, quits sending signals via ACTH to the adrenals to protect us from these stresses, and, without an ACTH signal, the adrenals shut down and the HPA Axis slides into adrenal insufficiency. At this point, we feel like we have been hit by a bus!
Adrenal Insufficiency and Poor Cerebral Spinal Fluid Circulation (glymphatic system).
An indirect, causal link between a generally "low HPA axis" and "poor cerebrospinal fluid (CSF) circulation" is an up-and-coming medical concept, though some conditions can feature both or be indirectly related through broader dysregulation of the central nervous system or metabolic processes. The most apparent connection between low adrenals and impaired CSF circulation stems from poor sleep. The brain contains large pools of cerebral spinal fluid (called ventricles), that fill with the metabolites, or waste from neuronal functioning during the day. It is during deep sleep that the glia (astrocytes) gather up this waste, and in particular, the tau proteins, and dumps them into the ventricles. From here, the ventricles circulate this dirty CSF into the lymphatic system for cleaning, and then the waste is eliminated in urine.
Most people with low functioning adrenals, have been under long-term stress and not slept well for years. So, the tau proteins and other toxic waste products build up within the brain and the neurons become “sick.” Side-effects from this include brain fog, depression, anxiety, and constant feelings of exhaustion. In the long term, this erodes the brain structure and becomes Alzheimer’s, Parkinson’s, and in athletes, Post-concussion Syndrome (Chronic Traumatic Encephalopathy). All of these conditions are common in that, sleep is severely impaired!
Audio-Visual Stimulation / Entrainment
Audio-Visual Stimulation / Entrainment (AVE) has been used with thousands of personnel in the military and first responders, such as police, firemen, and paramedics. The response rate has been excellent, but with the CSF Circulation Boosting (Pump) Session, we expect the results to be even better.
So far, one severe early-onset Alzheimer’s study participant made a complete recovery (Budzynski and Sherlin, 2011) and a Parkinson’s patient has made a near-complete recovery (in-house observations). Other Parkinson’s patients have shown remarkable improvements on sensory-evoked potentials (SEPs), which show the speed and efficiency at which stimuli flow through the brain; from sensory awareness to cognitive awareness (Lemay, 2025).
The CSF Pump session is a clever and simple concept. Using visual stimulation only, the arteries within the brain swell from the increased blood flow in response to the stimulation. In response, the brain must equalize intracranial pressure by pumping CSF out of the ventricles and into the cerebrospinal fluid for disposal into the glymphatic system. This time-period takes 15 seconds. Then the stimulation turns off. In response to the loss of stimulation, the arteries constrict over a 15-second period, in which the brain must bring CSF into the brain in order to balance intracranial pressure. This total process takes 30 seconds, in which 10% of the CSF gets exchanged for cleaner CSF. Over the course of one minute, 20% of the CSF may be exchanged. Adding audio increases this process and cleaning of CSF is more apparent.

A) Examples from the block-design visual stimulus. “On” is checkerboard. “Off” blocks are a gray screen.
B) The analysis extracted the CSF inflow signal measured in the fourth ventricle (blue) moving upwards (purple arrow) into the functional acquisition volume. The black line indicates an example position of the bottom slice of functional volume through the fourth ventricle, to enable upwards CSF flow detection.
C) Map in MNI space of widespread cortical activation in response to block-design checkerboard visual stimulus in n = 16 subjects in Experiment 3. Z-statistic values were thresholded at a level of 3.1 and corrected voxelwise for multiple comparisons (p < 0.05).
D) Left: Example placement of CSF ROI (blue) in the fourth ventricle in one subject in Experiment 1. Right: Average evoked CSF inflow signal locked to the visual stimulus across subjects in Experiment 1 (n = 6).
E) Left: Example placement of the CSF ROI in Experiment 2, in the upper ventricle and aqueduct. Right: Average evoked CSF inflow signal across subjects in Experiment 2 (n = 20).
F) (F) Left: Example placement of CSF ROI in Experiment 3, in the fourth ventricle. Right: Average evoked CSF inflow across subjects in Experiment 3 (n = 16). The black bar indicates “on” blocks. Shading indicates standard error across subjects.
Conclusion
Understanding the HPA axis is essential for recognizing how stress affects the body and for developing effective treatments for stress-related disorders. These disorders are not only physical, but also social, emotional, and cognitive. Supporting the HPA Axis through stress management techniques, therapy, and lifestyle changes can help maintain its proper function and overall health.
AVE is a very helpful technique for preventing or reversing adrenal insufficiency. It does so mainly by inducing a meditative style of dissociation, in which the person under stress enters into a deep trance, quits thinking about his/her stressors, and enters into complete and deep relaxation during the session and often for several hours following the AVE session. This gives the adrenals a chance to recover, and the struggling person gets some sense of vitality and health back for a period of time. In addition to this, with regular use, the person has improved sleep and a glymphatic system that improves with the deep sleep and cleans the CSF. If a person is in serious trouble with neurological struggles, such as brain fog, depression, and continued disrupted sleep, then using the CSF Pump session during the day (and often in addition to a Mood Booster session) will be the next best option.
Budzynski & Sherlin (re-edited by Dave Siever). (2017): AVE_Case_Study_on_Alzheimer-Budzynski_Sherlin-2011.pdf
Geeky Medics. (2025): 💬1 - How the Adrenal Axis Works | Geeky Medics
Lemay, J. (2025). In progress.
Siever, D., (2025). Audio-visual entrainment and dementia: CSF Circulation Boosting Sessions
Williams, et al. (2023). Neural activity induced by sensory stimulation can drive large-scale cerebrospinal fluid flow during wakefulness in humans. PLoS Biol. 21(3): e3002035.